α-synuclein (α-syn) is a protein that plays a significant role in the pathogenesis of Parkinson’s disease (PD) and mutations in this protein, including the A53T mutation, are associated with early-onset PD.
In healthy neurons, α-syn is thought to be involved in the regulation of synaptic function and neurotransmitter release. However, in PD, α-syn undergoes misfolding and aggregation, forming insoluble protein deposits known as Lewy bodies. These aggregates are a pathological hallmark of PD and aggregated or oligomeric forms of α-syn are suggested to contribute to the progressive degeneration of dopaminergic neurons in the substantia nigra.
In addition, dysregulation of the autophagy pathway has been observed in the brain of PD patients and PD animal models, indicating the significant role of autophagy in this disease.
To assess different aspects of α-syn aggregation, seeding and autophagy, the following assays are set up at Scantox, which can be modified to be used within different cell systems, including immortalized cell lines, primary rodent or human iPSC-derived neurons.
–
Application of pre-formed fibrils (PFF) or oligomers to induce cell death or seeding
Recombinant human α-syn fibrils can be used to evaluate seeding properties and induce toxicity on primary cortical mouse neurons in vitro.
While monomeric human α-syn has no impact on cell viability, pre-formed wild type and A53T human α-syn fibrils have toxic effects on primary cortical mouse neurons. This toxic effect is exceeded by oligomeric isoforms (Figure 1A). Only A53T α-syn fibrils of the tested isotypes show seeding properties in cortical mouse neurons (Figure 1B), which can be assessed either via immunocytochemical (Figure 2) or biochemical assays utilizing the Mesoscale Discovery and WES platform for protein analysis (Figure 3).
Assessment of murine instead of human α-syn in this murine cell system avoids capturing signal of the applied seeds.
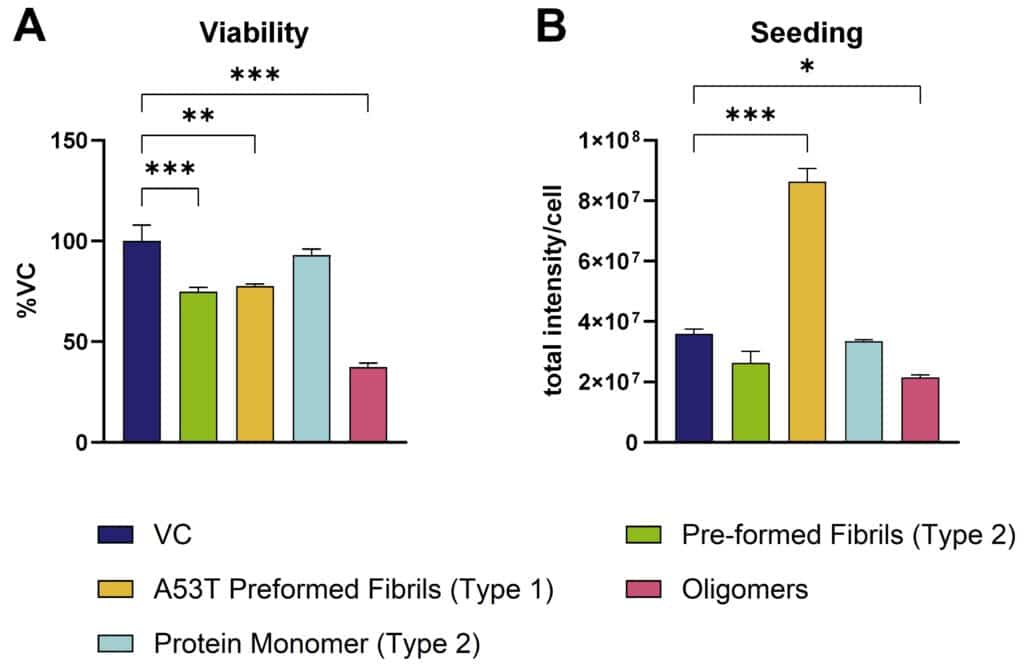
Figure 1: In vitro assessment of α-syn pre-formed fibrils on primary cortical mouse neurons. Toxicity (A) as well as seeding properties (B) of different pre-formed recombinant human α-syn species (StressMarq) on primary cortical mouse neurons. A: Neurons treated with α-syn species and assessed for cell viability by MTT assay. B: Neurons treated with α-syn species and immunocytochemically analyzed for murine α-syn. Data are presented as bar graphs with standard error of mean (SEM). For statistical analysis, One-way ANOVA with Dunnett’s post hoc test versus vehicle control (VC) was used. *p<0.05, **p<0.01, ***p<0.001.
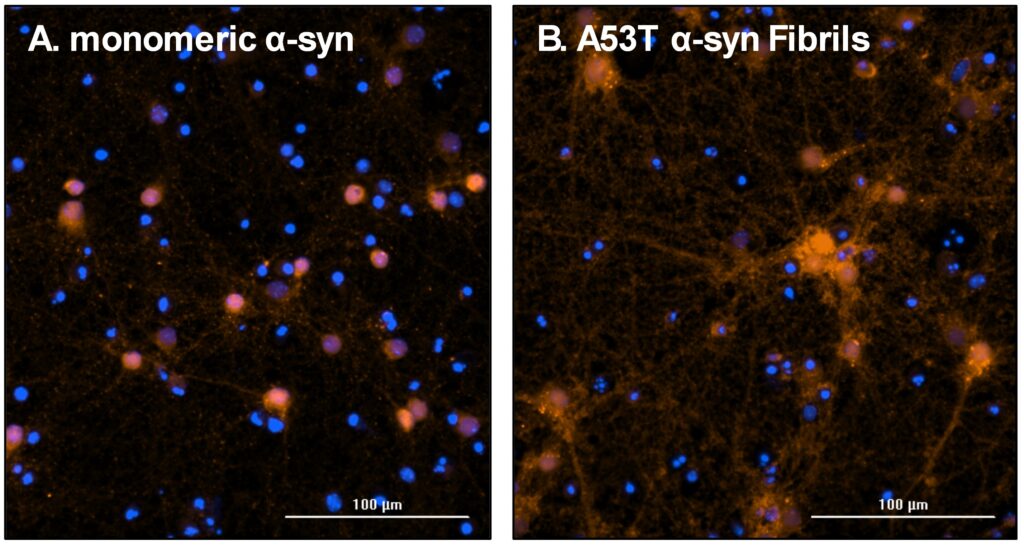
Figure 2: Representative images of endogenous murine α-syn accumulation after seeding with different pre-formed recombinant human α-syn species (StressMarq). Neurons were treated with monomeric (A) and A53T pre-formed fibrils (B) and after incubation immunocytochemically stained for murine α-syn. Nuclear stain DAPI = blue; murine α-syn = red; scale bar 100 µM.
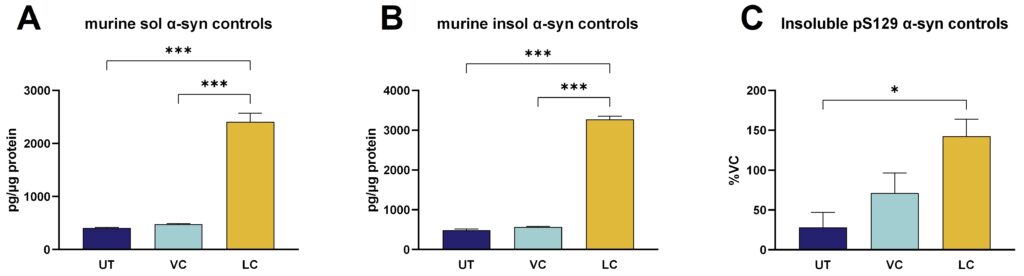
Figure 3: Murine soluble and insoluble α-syn as well as pS129 α-syn in PFF-stimulated primary cortical mouse neurons. Soluble murine α-syn (A) and insoluble murine α-syn (B) analyzed by Mesoscale Discovery platform and shown as pg/µg protein as well as insoluble α-syn phosphorylated at pS129 (C) analyzed by WES and shown as normalized signal in percent of the vehicle control (VC). Data are displayed as bar graphs with group mean + SEM (n=6 per group). UT: untreated, VC: vehicle control, monomeric α-syn-treated cells, LC: lesion control, PFF α-syn-treated cells. For statistical analysis One-way ANOVA followed by the Dunnett’s post hoc test versus lesion control (LC) was used. *p<0.05, ***p<0.001.
–
Assessment of α-synuclein aggregation and autophagy
The effect of experimental compounds on general α-syn aggregation and changes in autophagy can be assessed in stably transfected SH-SY5Y cells overexpressing wild type α-syn (SH-SY5Y-SNCA).
Differentiated SH-SY5Y-SNCA cells are incubated with developmental compounds or reference items and thereafter harvested for extraction of soluble and insoluble proteins.
The extent of α-syn aggregation (Figure 4) or expression of autophagy markers (Figure 5) can then be quantified using the MESO QuickPlex SQ 120MM platform or by immunocytochemistry.
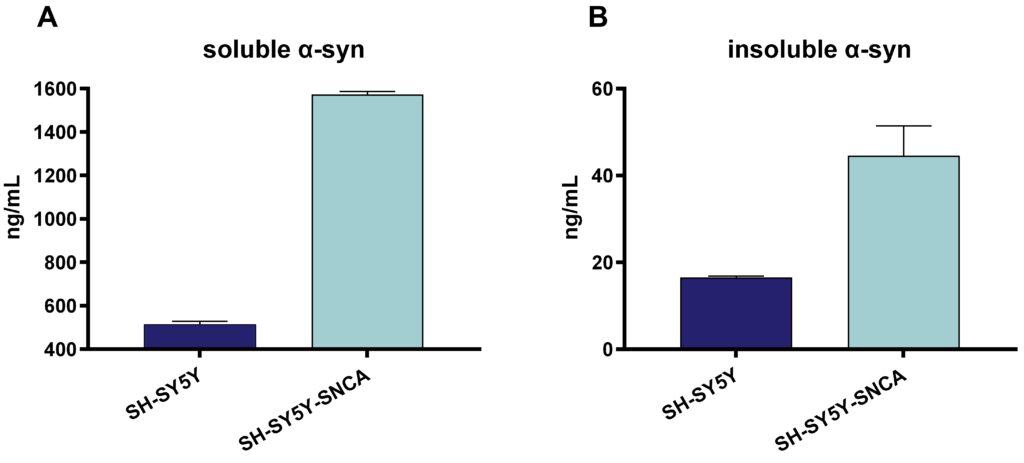
Figure 4: Human soluble and insoluble α-syn in SH-SY5Y cells and stably transfected SH-SY5Y-SNCA. Soluble α-syn (A) and insoluble α-syn (B) analyzed by Mesoscale Discovery platform and shown as ng/mL. Data are displayed as bar graphs with group mean + SEM (n=6 per group).
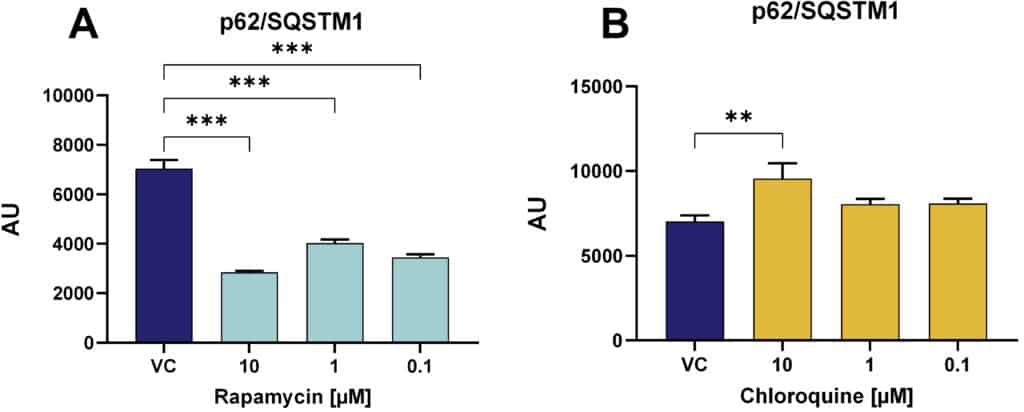
Figure 5: Quantification of the autophagy marker p62/SQSTM1 in SH-SY5Y-SNCA cells after different treatments. Effect of rapamycin (A) or chloroquine (B) on p62 levels in SH-SY5Y-SNCA cells analyzed by Mesoscale Discovery platform. Data are displayed as bar graphs with group mean + SEM (n=3-6 per group). VC: vehicle control. For statistical analysis One-way ANOVA followed by the Dunnett’s post hoc test versus VC was used. **p<0.01, ***p<0.001.
We are happy to evaluate the efficacy of your compound in these in vitro PD models!
