After evaluating main phenotypic traits of Fmr1-KO mice showing increased activity, hyperactivity, anxiety, and repetitive behavior (Behavior Newsletter) we now evaluated brain pathological hallmarks of young Fmr1-KO mice.
In Fmr1-KO mice, the Fmr1 gene on exon 5 is replaced by more than 200 CGG repeats with a neomycin resistance cassette. Animals thus present a valuable genetic tool to model the Fragile X syndrome, which is the most frequently inherited form of intellectual disability and the monogenic cause of autism spectrum disorder (ASD). After validating disease-relevant behavioral changes in Fmr1-KO mice, we now focussed our analysis on disease-specific biomarkers and evaluated different brain regions for levels of the serotonin transporter protein (5-HTT) as marker of the serotonin signalling pathway, parvalbumin (PV) as marker of interneurons, Iba1 as marker of microglia, and GFAP as marker of astrocytes.
Our results show that at the age of 7 weeks, 5-HTT levels are significantly increased in the caudate putamen of Fmr1-KO mice compared to C57Bl/6J mice (Figure 1B) while levels in the cortex and hippocampus are not altered (Figure 1A, C). Evaluation of PV levels in Fmr1-KO mice of same age resulted in significantly increased levels in the cortex (Figure 1E), while levels in the caudate putamen and hippocampus were unaltered (Figure 1F, G). Representative images of 5-HTT labelling in the hippocampus and PV labelling in the cortex are shown in Figure 1D and H.
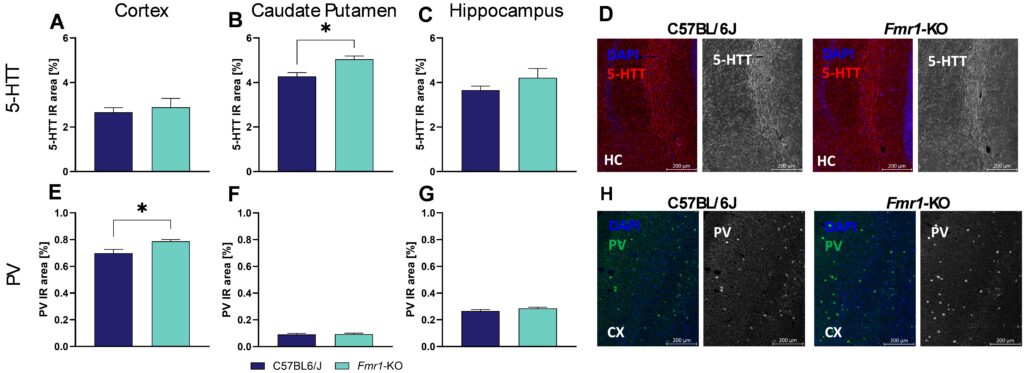
Figure 1: Quantification of serotonin transporter protein (5-HTT) and PV in 7 weeks old Fmr1-KO and C57BL/6J mice. 5-HTT expression levels (A-C) and parvalbumin (E-G) in cortex (A, E), caudate putamen (B, F), and hippocampus (C, G) as evaluated by immunoreactive (IR) area in percent. Representative images of DAPI (blue), and 5-HTT in the hippocampus (red, D) or PV in the cortex (green, H) of C57BL/6J and Fmr1-KO brain sections. Unpaired t-test. n = 5 per group. Mean + SEM. *p<0.05. CX, cortex; HC, hippocampus; PV, parvalbumin.
Quantification of Iba1 immunoreactivity in Fmr1-KO mice further showed significantly increased levels in the caudate putamen (Figure 2B) but not in the cortex and hippocampus (Figure 2A, C), indicating a severe increase in activated microglia as marker of neuroinflammation in the caudate putamen. Analysis of GFAP immunoreactivity in Fmr1-KO mice showed no changes in the cortex and caudate putamen (Figure 2E, F) but significantly increased levels of GFAP in the hippocampus (Figure 2G). Representative images of Iba1 labelling in the caudate putamen and GFAP labelling in the hippocampus are shown in Figure 2D and H.
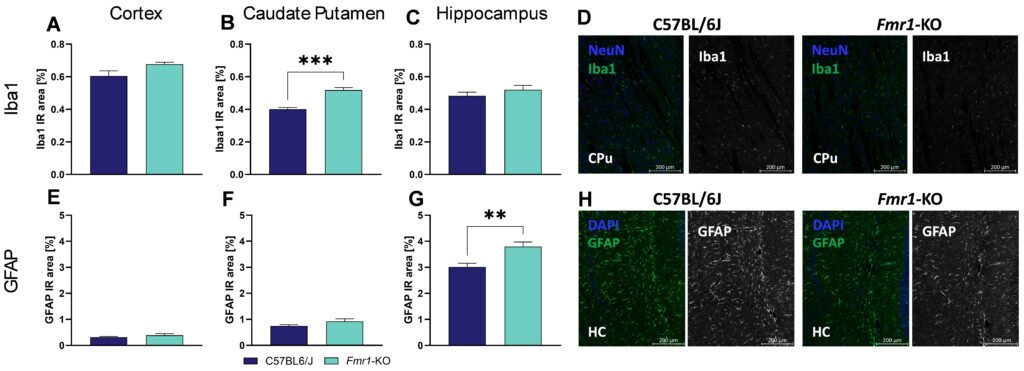
Figure 2: Quantification of Iba1 and GFAP in 7 weeks old Fmr1-KO and C57BL/6J mice. Iba1 (A-C) and GFAP (E-G) levels in cortex (A, E), caudate putamen (B, F), and hippocampus (C, G) as evaluated by immunoreactive (IR) area in percent. Representative images of NeuN (blue) and Iba1 (green, D) in the caudate putamen and DAPI (blue) and GFAP (green, H) in the hippocampus of C57BL/6J and Fmr1-KO brain sections. Unpaired t-test. n= 5 per group. Mean + SEM. **p<0.01; ***p<0.001. CPu; caudate putamen; HC, hippocampus.
In summary, these data show that Fmr1-KO mice present disturbed serotonin signaling and interneuron network as well as a clear neuroinflammatory pathology. Interestingly, all observed pathologies are highly region-specific, making this mouse an ideal model to analyze new ASD compounds for their efficacy.
Contact us today to get your Fmr1-KO mouse study started!









