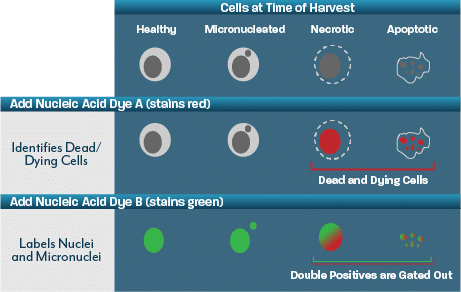
Conducted in a miniaturised format and using flow cytometry to score both micronuclei and cytotoxicity, the flow micronucleus assay provides a rapid prediction of the in vitro micronucleus test. The miniaturised format enables the study to be performed using far fewer test items than an OECD 487 study and results in more responsive delivery timelines.
Advantages for Screening
- High throughput
- Clastogen & Aneugen detection
- Quick, cost-effective, high-quality results
| Test Cell Line | TK6 |
| Metabolic activation | Typically, induced rat liver S9, other sources available. 3 hours (+S9) and 24 hours (-S9) Short +S9 Long –S9 2 arm study |
| Typical test item requirements | 20 mg (ICH S2(R1)) - 50 mg (OECD 487) |
| Study Duration | < 3 Weeks |
| Endpoint | Micronucleus Formation / Chromosomal Damage |

