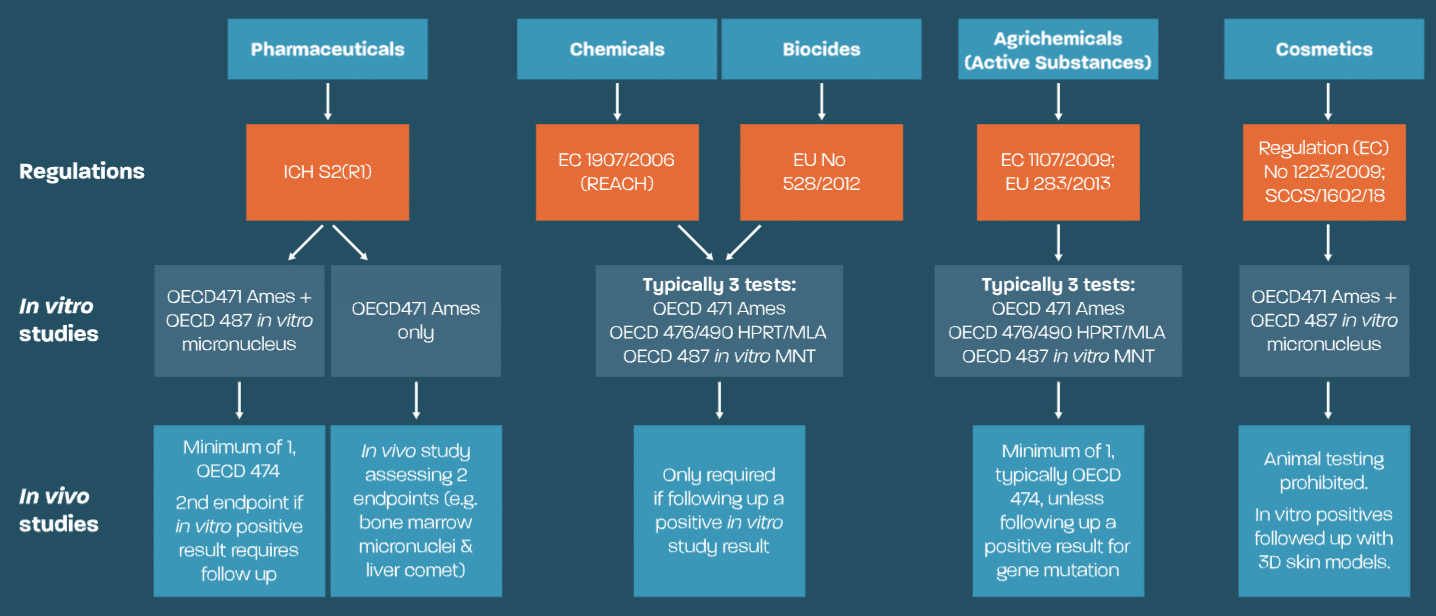
Scantox offers GLP OECD regulatory genotoxicity studies for assessing chemical safety across global industries, covering endpoints detection for mutagenic, clastogenic, and aneugenic mechanisms in our GLP genotoxicity lab.
Service categories:
Comprising a scientific team of experts from pharmaceutical, agrochemical, and CRO backgrounds, Scantox has a wealth of experience in delivering regulatory genotoxicity testing that adheres to genotoxicity studies guidelines. Our laboratory facilities at Alderley Park are GLP-compliant and purpose-designed for genotoxicity testing. We are committed to providing high-quality genotoxicity services and facilitating safety assessment for gene mutation structural and numerical chromosomal damage.

