The RNA-binding protein TDP-43 is strongly linked to neurodegenerative diseases like ALS and FTLD.
Several studies have shown that cytoplasmic TDP-43 aggregates co-localize with stress granule markers. Stress granules (SGs) are cytoplasmic inclusions that repress translation of a subset of RNAs during cellular stress.
Since it was shown that SG formation contributes to accumulation of TDP-43, inhibition of SG formation and/or recruitment of TDP-43 to SGs are pathways that are currently in the focus of ALS research.
To be able to support this research, we have set up a respective in vitro model in TDP-43 overexpressing neuroblastoma cells.
We therefore treated cells with the well-described SG inducer sodium arsenite (SA) and investigated TDP-43 aggregation in soluble and insoluble protein fractions via proteinsimple WES technology (Fig 1) as well as SG formation and TDP-43 recruitment via Immunocytochemistry (Fig. 2). WES analysis showed a strong shift from soluble to insoluble TDP-43 species upon SA treatment (Fig. 1).
Immunocytochemical staining for the SG marker G3BP revealed substantial SG formation in SA treated cells compared to the respective vehicle control (Fig. 2). As previously shown, cycloheximide can counteract SG formation (Fig. 2 bottom row).
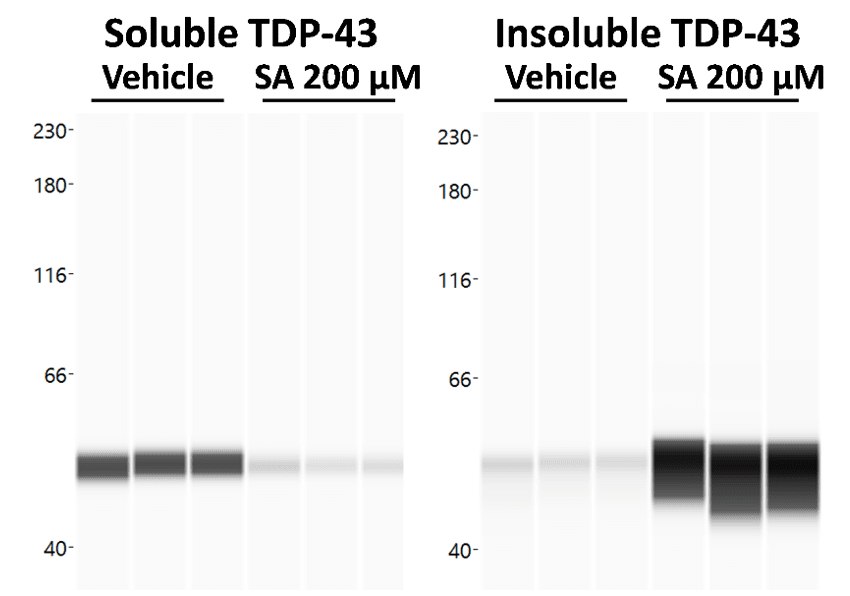
Figure 1. Effect of Sodium Arsenite (SA) treatment on soluble and insoluble TDP-43 levels. Cells were harvested after SA or vehicle treatment and a soluble and insoluble protein fraction were separated. Both fractions were analyzed for TDP-43 on proteinsimple WES. WES lane view of TDP-43 signal in soluble and insoluble fraction.
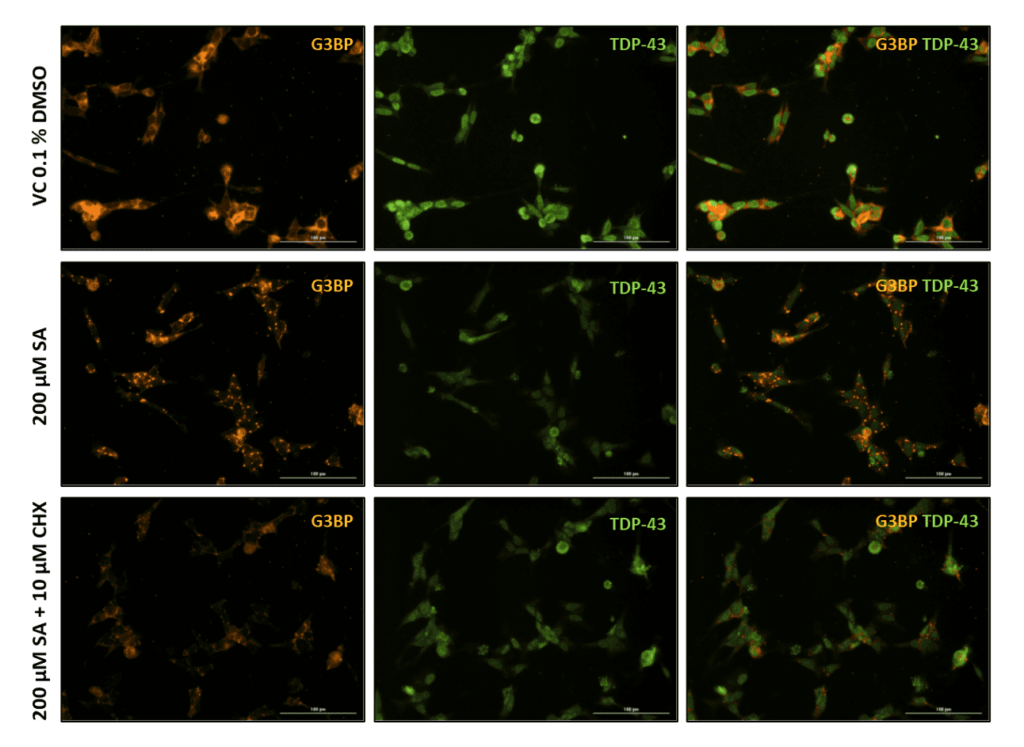
Figure 2. Representative images of Sodium Arsenite and Cylclohexamide treated SH-TDP-43 cells. Vehicle (VC 0.1% DMSO), 200 µM Sodium Arsenite (SA) and SA lesioned plus 10 µM Cycloheximide (CHX) treated SH-TDP-43 cells stained for the stress granule marker G3BP (orange) and human TDP-43 (green). Scale bar 100 µm.
Contact us today to get your in vitro study started!









