In recent years, gene and cell therapies have revolutionized treatment options for previously incurable diseases. Their thorough testing in preclinical studies using cells, tissues and animals is crucial before they reach the clinics to assess therapeutic effects and potential adverse side effects. Preclinical studies are key to demonstrating efficacy, ensuring successful translation to patients and minimizing safety concerns. At Scantox, we specialize in customized preclinical gene therapy studies to support your specific needs, including comprehensive analysis of tissue samples from gene therapy research, to drive successful and save treatment development.
Efficacy
After administering gene therapy to preclinical animal models, proper evaluation of treatment efficacy is of utmost importance. Efficacy on mRNA level can be evaluated by RT-PCR, qRT-PCR, fluorescent in situ hybridization (FISH), while efficacy on protein level can be evaluated by western blotting, automated western blotting (WES), MesoScale Discovery (MSD) immunosorbent assay, and immunofluorescent labeling. FISH can also be applied for vector localization. For instance, intrathecal administration of an antisense oligonucleotide (ASO) to rats results in a significant reduction of target mRNA levels, as confirmed by qRT-PCR. However, protein levels, assessed via the MSD assay, were less reduced (Figure 1). This example underscores the importance of employing multiple analytical methods to provide a comprehensive view of gene therapy outcomes. (Figure 1).
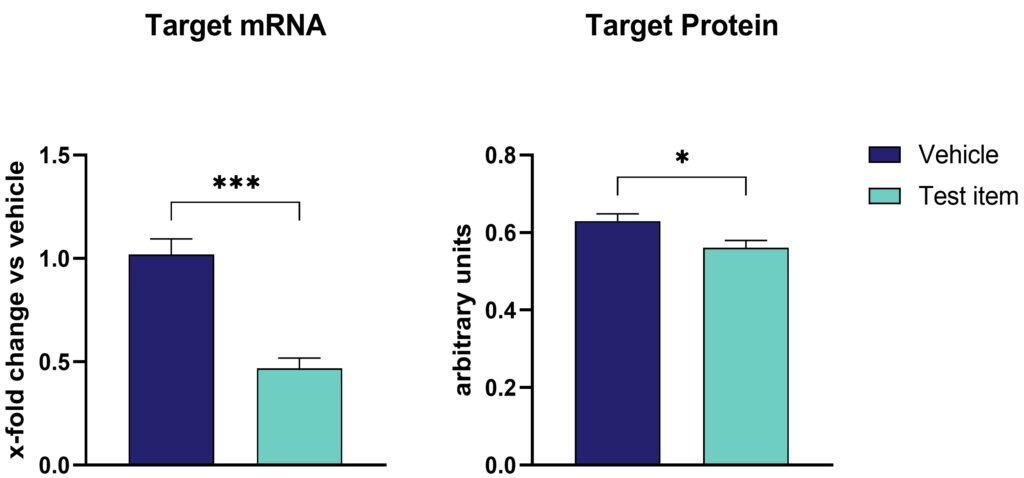
Figure 1: Target expression on protein and mRNA level upon intrathecal antisense oligonucleotide (ASOs) injection in rats. (A) Target mRNA expression evaluated by qRT-PCR and (B) target protein levels analyzed by MesoScale Discovery assay in total brain homogenates treated either with vehicle or test item. Unpaired t-test. n=8 per group. Mean + SEM; *p<0.05, ***p<0.001.
Immunofluorescent labeling of the target protein is specifically of interest for quantitative comparisons in the brain after intracerebral injection, such as AAV-A53T- α-synuclein injections as model of Parkinson’s disease (Figure 2).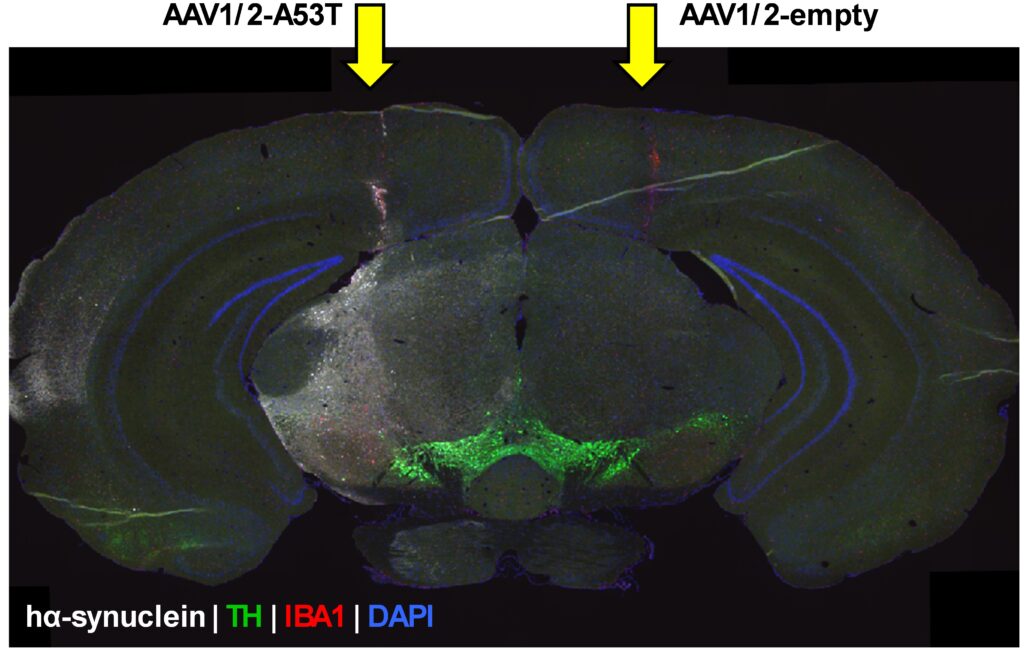
Figure 2: Immunofluorescent signal of human α-synuclein in a coronal whole brain tissue section in an intracerebrally AAV-A53T α-synuclein- or empty vector-injected mouse model. hα-synuclein: Human α-synuclein, TH: Tyrosine hydroxylase, IBA1: Ionized calcium binding adaptor molecule 1, DAPI: 4′,6-diamidino-2-phenylindole.
Administration Routes
The route of administration plays a critical role for the success of gene delivery, as it affects distribution, expression levels, and potential side effects. Scantox offers various administration routes to treat preclinical animal models, each with its own advantages and disadvantages. The selection of the appropriate route should be based on factors such as the host, the test compound, and the specific research objectives (Table 1). New application routes are constantly established and validated such as the most recent cisterna magna injection in rodents.
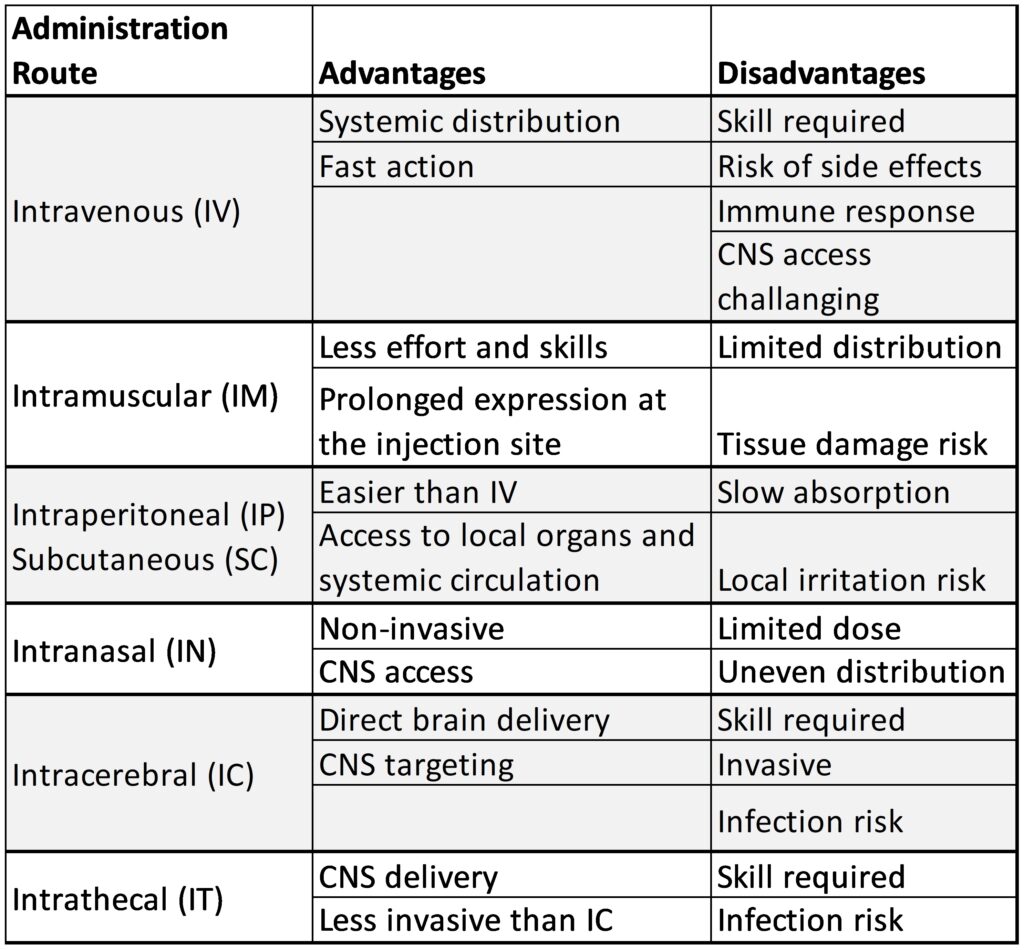 Table 1: Advantages and disadvantages of the current administration routes used for gene therapy studies that are available at Scantox.
Table 1: Advantages and disadvantages of the current administration routes used for gene therapy studies that are available at Scantox.
Toxicity
Various viruses can access the CNS causing severe neurological disease or death. Therefore, it is essential to identify therapeutic compounds capable of inhibiting viral replication in neurons or preventing virus-induced neuronal cell death. To test toxicity in vitro, Scantox offers the MTT assay, a colorimetric test to determine cell cytotoxicity by providing information about cell proliferations, viability and toxicity (Figure 3).
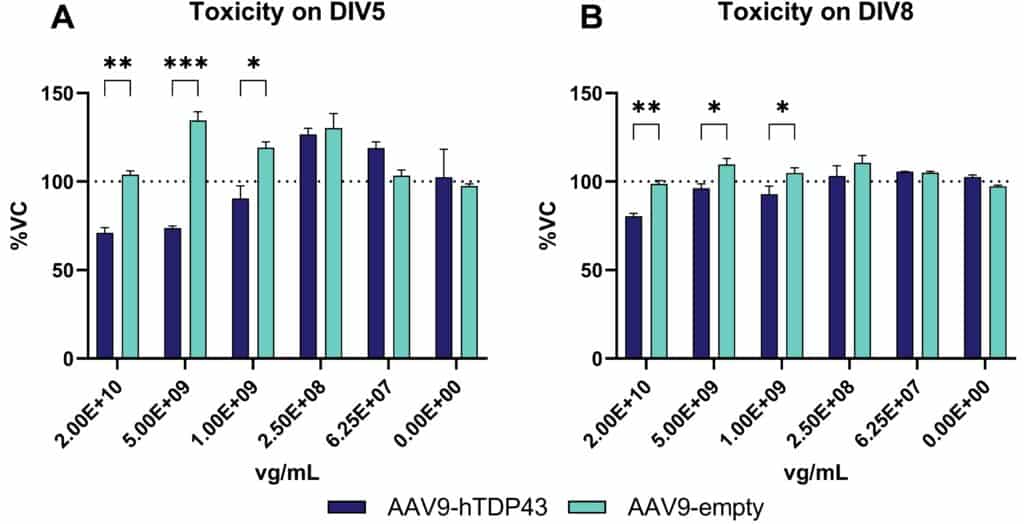
Figure 3: Toxicity of AAV9-hTDP43 and AAV9-empty over time using the MTT assay for cell viability. Cells were treated with AAV9-hTDP43 or AAV-empty at five concentrations. After 5 (A) and 8 days (B), cell viability was measured using the MTT assay. Two-way ANOVA followed by the Bonferroni`s post hoc test. n=3 per group, Mean±SEM *p<0.05, **p<0.01, ***p<0.001. DIV: days in vitro.
Scantox also offers in vivo toxicity and genetic toxicity testing that are not presented in this Science News.
Scantox capabilities enhance the precision and effectiveness of gene therapy for improved therapeutic outcomes. Our services will thus foster your cell and gene therapy research in the field of neurodegenerative and rare diseases.
Contact us today to get your gene therapy study started!