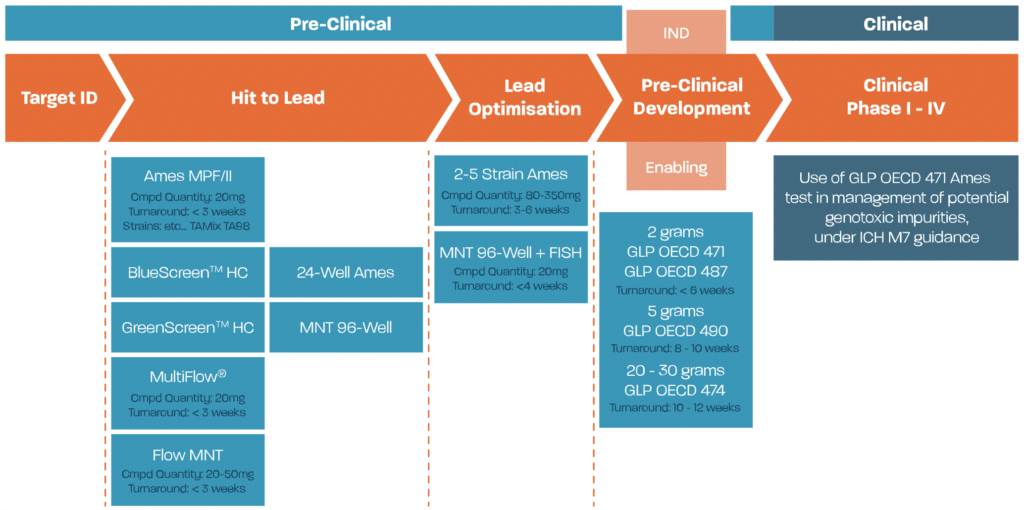
Genotoxicity assessment, including genetic toxicology testing, is an essential component of safety assessment for all types of chemicals, such as pharmaceuticals, pesticides, food substances, bulk chemicals, and cosmetics. Genotoxicity/DNA damage, manifested through mutagenic (point mutation), clastogenic, aneugenic, or secondary mechanisms, can be a significant health concern as substances causing these effects may do so at very low doses. Changes to DNA can result in severe diseases such as cancer, and altered DNA may also be passed to offspring. Consequently, detecting genotoxic liability early in a chemical’s development is very important to avoid costly late-stage failures.
Scantox has extensive experience conducting and evaluating genotoxicity testing for early-stage screening and supporting regulatory submissions. This includes using OECD guideline GLP studies and screening studies optimised for testing with very low amounts of test substance, often with additional options to help determine the mode of action.