During development, neurons become assembled into functional networks by growing out axons and dendrites, called neurite outgrowth, that synaptically connect to other neurons. Strikingly, neurons retain their capacity for growth and synapse formation in the adult brain. Stimulation and acceleration of neurite outgrowth is important to restore neuronal function after injury or in neurodegenerative diseases. A promising strategy for drugs targeted against neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease is to promote the regeneration of neurite outgrowth. Thus, new therapies are focusing on the identification of molecules that affect neurite outgrowth. For this purpose, Scantox provides cell culture models to assess the neurotrophic effects of your developmental compounds.
Neurite Outgrowth Solution 1
Primary mouse/rat hippocampal or cortical neurons are treated with compounds for 48 h on DIV1. After treatment, neurite outgrowth is assessed over time using the IncuCyte® label-free Live-Cell Analysis System. Live-cell analysis and treatments can be extended, depending on the scientific question.
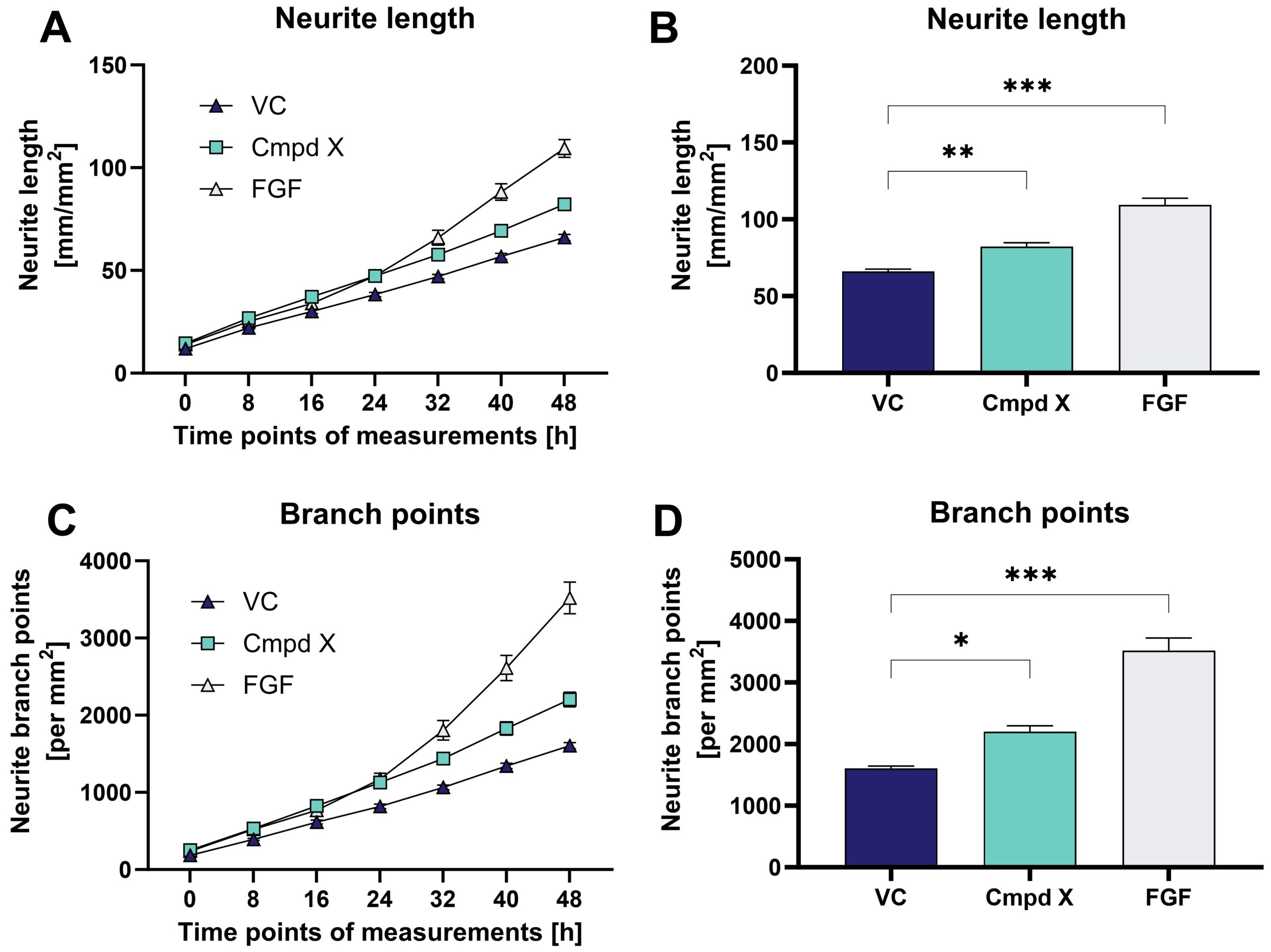
Figure: Effects of compound X (Cmpd X) and fibroblast growth factor (FGF) on neurite outgrowth and branching of primary mouse hippocampal neurons over time. Data are displayed as curve over time (A, C) or bar graphs (B, D) after 48 h treatment with group means + SEM (n=6 / group). One-way ANOVA followed by Dunnett‘s multiple comparison post hoc test compared to vehicle control (VC); *p<0.05; **p<0.01; ***p<0.001.
Neurite Outgrowth Solution 2
Primary mouse/rat hippocampal or cortical neurons are treated with compounds for 48 h on DIV1. Cells are subjected to indirect immunofluorescence analysis using anti-β-tubulin III antibody and imaged in Cytation5 multimode reader (Biotek) with imaging module at 10x magnification of 4-6 images per well. The following parameters using automated image-based analysis are determined:
- Total neurite outgrowth
- Longest neurite length
- Number of branches
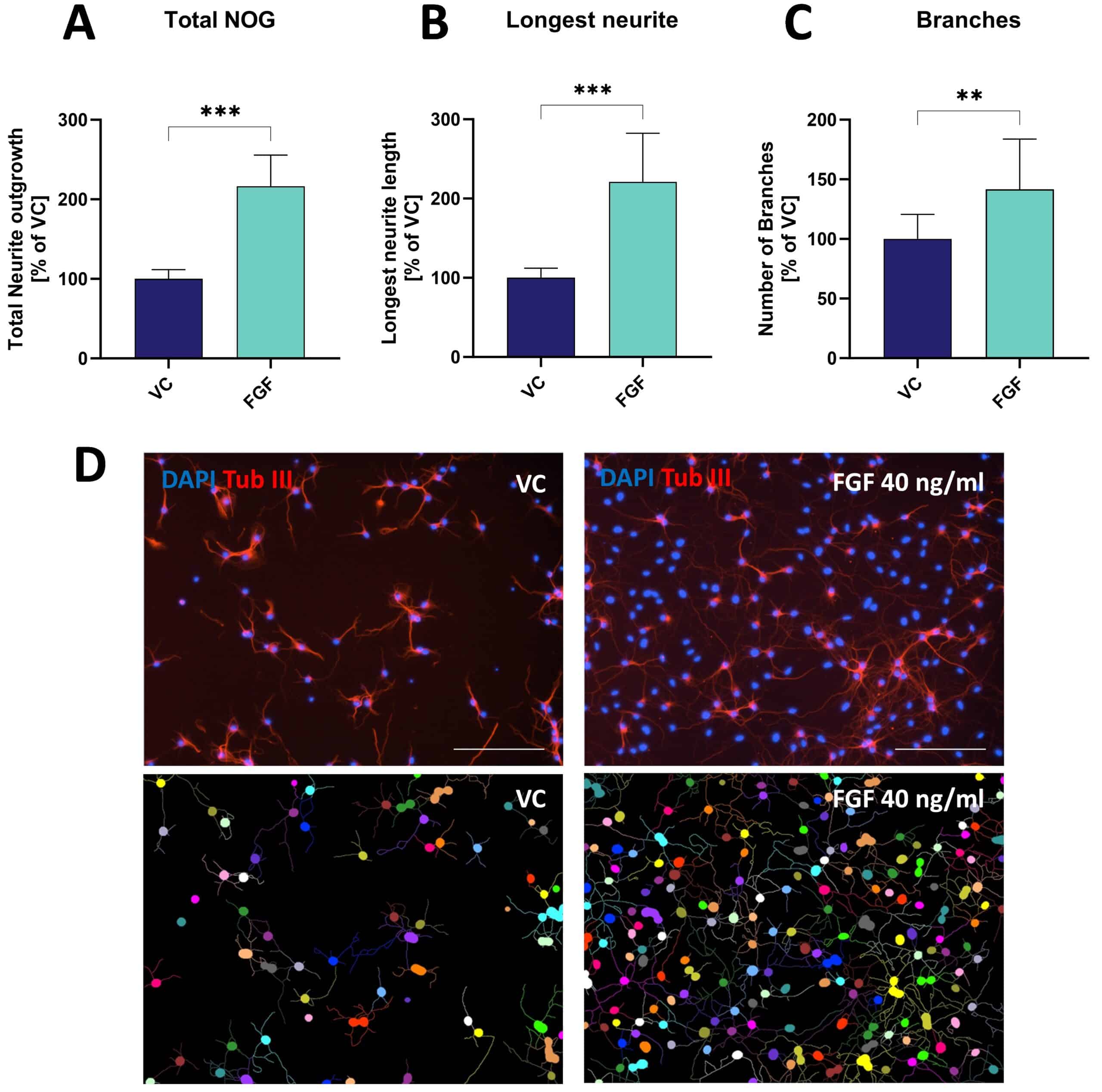
Figure: Effects of fibroblast growth factor (FGF) on neurite outgrowth (NOG) and branching of primary mouse hippocampal neurons. Data are displayed as bar graphs (A-C) after 48 h treatment with group means + SEM (n=6 / group). Unpaired t-test; **p<0.01; ***p<0.001. D: Representative images of NOG analysis. Cells treated either with vehicle (VC) or FGF were stained for β-tubulin III. Images before (top row) and after analysis (bottom row). Scale bar: 200 µM.
Neurite Outgrowth Solution 3
Microfluidic chambers (MFCs) can provide unique insights into the axonal compartment independent of the soma. We thus offer a model of axonal injury and re-growth in MFCs using dissociated dorsal root ganglion (DRG) neurons from adult wild type mice. Already 3 days after seeding neurons into the somal side of the MFC, axons start to cross the microgrooves. After 5 days in culture, axons have completely crossed the grooves and built a beautiful network on the axonal side (Fig 1 A). Neurons can then be axotomized by fast removal of the medium from the axonal compartment (Fig. 1 B).
Developmental compounds can be selectively applied to either axonal or somal side alone, by using a hydrostatic pressure difference between the somal and the axonal chamber.
The effect of NGF application to the axonal side in comparison to vehicle-treated cells was quantitatively analyzed, showing a significant increase of branching and axonal length, while no impact on the number of crossing axons could be observed (Fig.2).
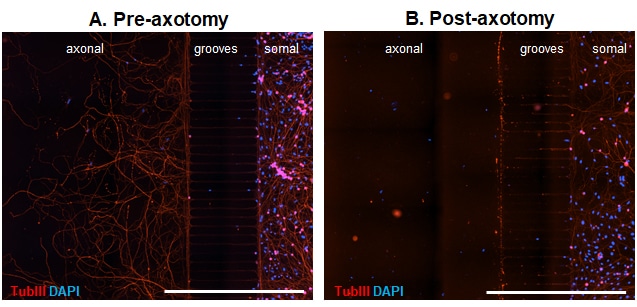
Figure 1. Representative images of DRG neurons from adult wild type mice in microfluidic chambers. DRG neurons were cultured until DIV5 and fixed before (A) and after (B) mechanical axotomy. Tubulin III (TubIII) labeling to visualize neurons and their extensions and DAPI to counterstain for nuclei. Crossing axons on the axonal side before axotomy (A) and removal of axons by axotomy (B). Scale Bar 1,000 µm.
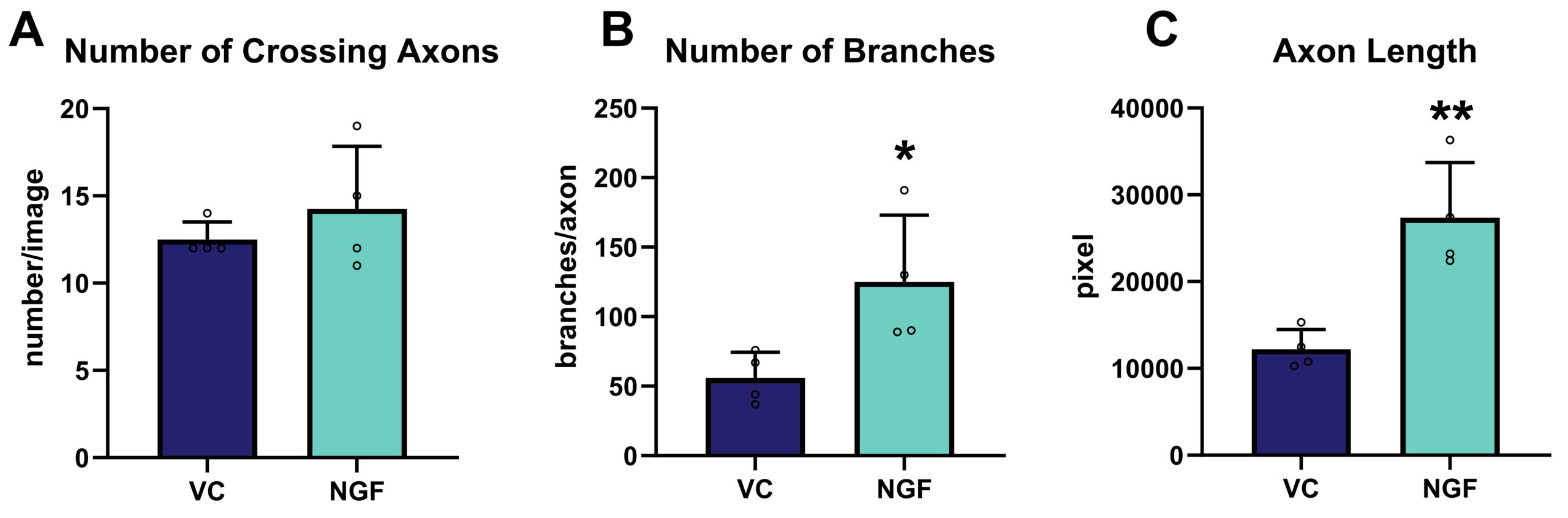
Figure 2. Effect of NGF treatment on axonal re-growth 24 h after axotomy. The following marker were assessed using image-based quantification of total number of crossing axons (A), the number of branch points per crossing axon (B) and the total length of all axons (C); n = 4 wells per group. Unpaired t-test; *p<0.05; **p<0.01. VC: vehicle control; NGF: nerve growth factor.


