Frontotemporal dementia (FTD), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) are all neurodegenerative diseases with one thing in common: abnormalities in tau, a key structural protein in the brain. Now, researchers at UC Santa Barbara may have discovered a way to interrupt the formation of neurofibrillary tangles that are made of misfolded tau protein. Though the research is still in the early stage, it could pave the way for groundbreaking therapeutic interventions targeting neurodegeneration.
How Tau Protein Interferes with Brain Function
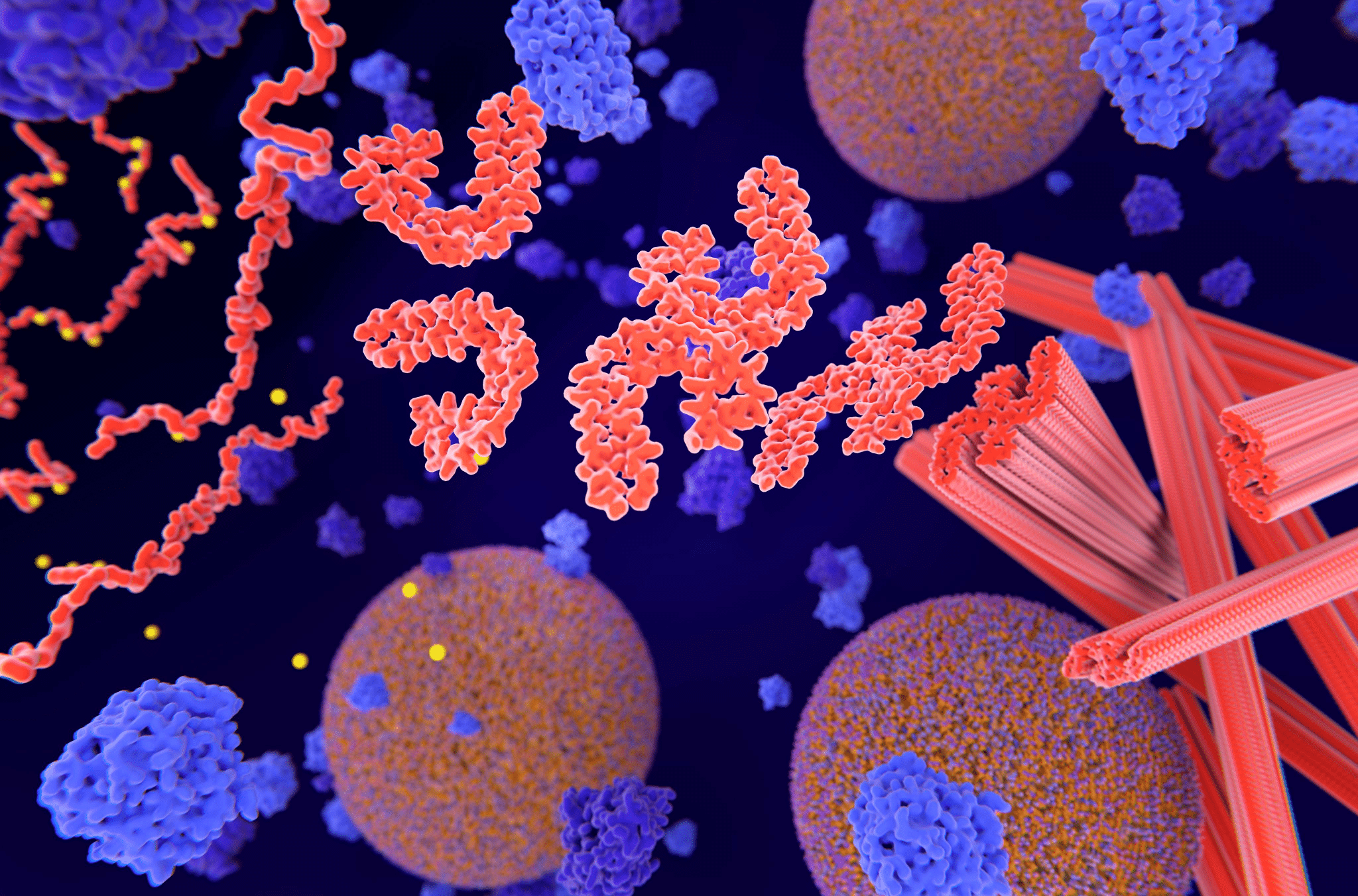
Tau protein plays a key role in brain health, helping stable cells form and maintain their structure while also facilitating nutrient transport in the brain. Unfortunately, abnormal tau — specifically, tau that has misfolded, damaging its structure and creating a sticky, tangled surface — contributes to a number of neurodegenerative conditions by forming neurofibrillary tangles. Individual misfolded tau proteins often have a domino effect, directing normal tau proteins to misfold and creating an accumulation of malfunctioning proteins. Over time, this accumulation can interfere with brain function and drive devastating neurodegenerative disorders, called tauopathies.
With this in mind, the UC Santa Barbara research team asked a question: what if we could stop tau from misfolding before it even starts?
Understanding Tau Protein Irregularities
Two forms of tau are generally associated with neurodegenerative diseases: a shorter “three-repeat” (3R) version, and a longer “four-repeat” (4R) version. Conditions like FTD, PSP, and CBD specifically involve the accumulation of 4R tau.
To understand how 4R tau begins to misfold, the UC Santa Barbara researchers combined cell culture experiments with advanced techniques, including transmission electron microscopy and molecular dynamics simulations. Through careful study, the team was able to identify a unique “hairpin” structure present in 4R tau. Within that structure, the researchers found a sticky segment called PHF6 — sticky in the sense that it can bind and stack other tau proteins into larger formations, driving the accumulation of potentially misfolded proteins. The researchers hypothesized that interfering with PHF6 could reduce the chance of widespread tau protein malformation and accumulation.
Targeting PHF6
Finally, the team needed to identify a substance that could bind to PHF6 to create a path for future research. The team flagged nanobodies — small fragments of antibodies — synthesized from the blood of animals in the Camelidae family, such as llamas. Camelid nanobodies are now thought to be a potential vehicle to help therapeutic interventions bind to the PHF6 region, thereby inhibiting the aggregation of tau. Moving forward, the team will scrutinize camelid nanobodies in hopes of identifying the precise factor that makes the nanobodies bind to PHF6.
_____
While the UC Santa Barbara team’s research is still in early phases, the team has identified two crucial factors to drive therapeutic interventions: first, the precise 4R area to target; second, a potential vehicle — the nanobodies — which could allow interventions to bind to the target.
The researchers still have a long way to go before identifying a working therapeutic solution to inhibit the formation of neurofibrillary tangles. In the future, the team plans to test the technology using animal models, ideally working toward a better understanding of how to disaggregate tau or even prevent its aggregation altogether. With ongoing research, the scientific community could be one step closer to clearing dysregulated proteins, improving treatment and quality of life for countless individuals with neurodegenerative conditions.
Scantox is the leading Nordic preclinical GLP-accredited contract research organization (CRO), delivering the highest grade of pharmacology and regulatory toxicology services since 1977. Scantox focuses on preclinical contract research services, supporting pharmaceutical and biotechnology companies with their drug development projects. Core competencies include explorative and efficacy studies, PK studies, general toxicology studies, local tolerance studies, wound healing studies, and vaccines. For more information about Scantox, visit https://scantox.com.