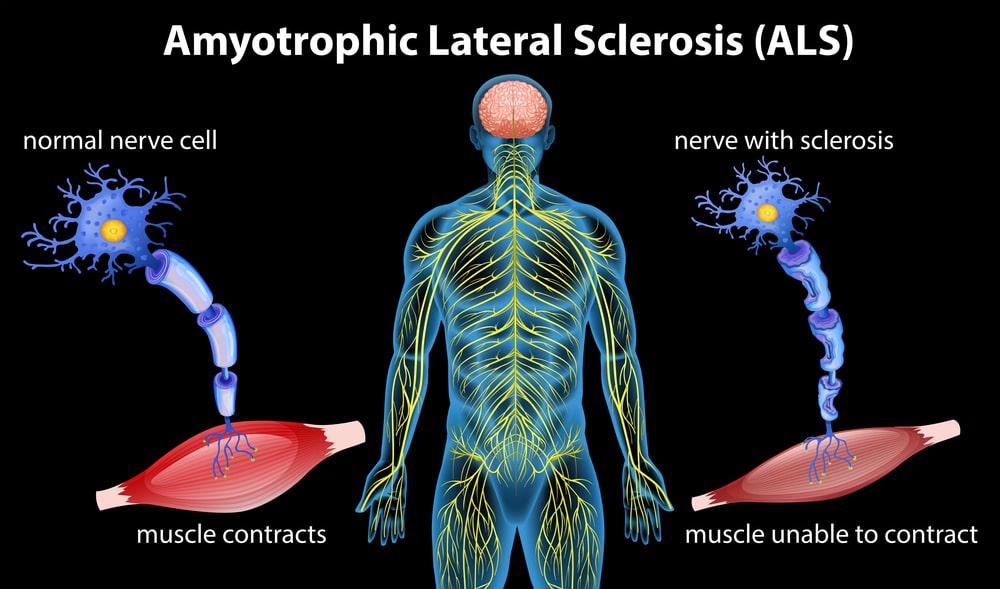
Amyotrophic lateral sclerosis, or ALS, is a progressive neurodegenerative disease affecting up to 30,000 people in the United States, and another 5,000 people are diagnosed every year. The disease damages and kills motor neurons in the brain and the spinal cord, eventually causing muscle weakness and paralysis. While there is currently no cure for ALS, Science Daily reports that recent research using ALS mouse models could shed light on future treatment options.
Lipid Metabolism in ALS Patients
As mentioned above, patients with ALS eventually lose most of their muscle control because of damaged motor neurons in the brain and spine. Most ALS patients can, however, still control their eye movements, which are guided by ocular neurons. With this in mind, a Johns Hopkins research team sought to explore the differences between disease-free ocular neurons and the damaged spinal neurons. To do that, the team evaluated active genetic pathways in individuals with ALS. Interestingly, researchers discovered increased activity in genes that control lipid metabolism, or the process during which cells process fat. After this discovery, the question was: What does lipid metabolism in nerve cells have to do with ALS symptoms?
Assessing Ocular and Spinal Neurons
To evaluate lipid metabolism in ALS patients, the researchers assessed samples of both ocular neurons and spinal motor neurons. The researchers took samples from 17 people with ALS, as well as six people without the condition. After carefully evaluating both sets of neurons, the researchers concluded that the spinal motor neurons of the people with ALS contained completely different amounts and types of lipids than the relatively unaffected ocular neurons. One specific pathway stood out in the ALS patients: arachidonic acid, an omega-6 fatty acid that controls the body’s inflammatory response. This acid is known to spur the body’s inflammatory processes – those needed to repair wounds, for example. Upon discovering high levels of arachidonic acid in ALS patients, the researchers wondered if reducing levels of arachidonic acid in ALS patients could serve to ease ALS symptoms.
How Researchers Created ALS Mouse Models
To explore arachidonic acid’s effect on ALS patients, researchers created a mouse model. First, they gathered a group of mice bred to develop the biological hallmarks of ALS. Researchers then reduced the mice’s arachidonic acid production using the anti-inflammatory compound caffeic acid. Researchers say that by tamping down the arachidonic acid pathway, they were able to “reduce the condition’s muscle-weakening symptoms in the mice,” which displayed a 20 to 25 percent increase in grip strength and an expanded lifespan of several weeks.
Findings
The Johns Hopkins researchers made several important determinations during the course of this research. First, unregulated arachidonic acid can lead to dangerous levels of inflammation, potentially destroying neural tissue. Similarly, tamping down arachidonic acid pathways may be an effective way to lessen the severity of ALS symptoms. However, caffeic acid, the anti-inflammatory compound used in the study, isn’t necessarily safe for human use. “Some reports have indicated potentially harmful side effects, including cancers and gut problems,” Science Daily writes. Either way, these findings may well prove valuable in the quest for a cure for ALS.
_____
Although researchers still need to further evaluate the differences between ocular and spinal neurons, the ALS mouse models used during this study may have groundbreaking implications for future ALS treatments.
Scantox is a part of Scantox, a GLP/GCP-compliant contract research organization (CRO) delivering the highest grade of Discovery, Regulatory Toxicology and CMC/Analytical services since 1977. Scantox focuses on preclinical studies related to central nervous system (CNS) diseases, rare diseases, and mental disorders. With highly predictive disease models available on site and unparalleled preclinical experience, Scantox can handle most CNS drug development needs for biopharmaceutical companies of all sizes. For more information about Scantox, visit www.scantox.com.